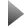76
nanotimes News in Brief
11-06/07 :: June/July 2011
Sensors // Nanowire-based Sensors Offer Improved Detection of Volatile Organic Compounds
© Text: NIST
George Mason University and the University of Maryland has made nano-sized sensors that detect volatile organic compounds – harmful pollutants released from paints, cleaners, pesticides and other products – that offer several advantages over today‘s commercial gas sensors, including low-power room- temperature operation and the ability to detect one or several compounds over a wide range of concen- trations.
A
The recently published work is proof of concept for a gas sensor made of a single nanowire and metal oxide nanoclusters chosen to react to a specific orga- nic compound. This work is the most recent of seve- ral efforts at NIST that take advantage of the unique properties of nanowires and metal oxide elements for sensing dangerous substances.
Modern commercial gas sensors are made of thin, conductive films of metal oxides. When a volatile or- ganic compound like benzene interacts with titanium dioxide, for example, a reaction alters the current running through the film, triggering an alarm. While thin-film sensors are effective, many must operate at temperatures of 200° C (392° F) or higher. Frequent
Scanning electron microscope image of a gas sensor segment fabricated of a semiconducting nanowire of gallium nitride. The nanowire of less than 500nm across is coated with nanoclusters of titanium dio- xide, which alters the current in the nanowire in the presence of a volatile organic compound and ultravi- olet light. © NIST
team of researchers from the U.S. National Institute of Standards and Technology (NIST),
heating can degrade the materials that make up the films and contacts, causing reliability problems. In addition, most thin-film sensors work within a narrow range: one might catch a small amount of toluene
 Previous Page
Previous Page