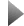82
nanotimes Patents
tic species covalently attached to the diamondoid. Hereby, the diamondoid molecule fulfills the role of an electron donor.
It has been discovered that rectification at the molecular level can be achieved by combining a diamondoid molecule with an electron acceptor – most notably an aromatic one. Depending upon the particular combination of diamondoid molecule with electron acceptor, these molecules may act as rectifiers, resistors, p-n junctions, or a combination thereof.
According to the present invention, anything that can serve as an electron acceptor (or electron- withdrawing group) is suitable as “N-type” material. Such materials include, for instance, C60 (fullerene), carbon nanotubes, or conducting polymers. A mole- cular functionalization on the diamondoid itself, such as –NO2, -CN, halogens (F, Cl, Br, I), alkenes, etc., is also suitable as “N-type” material. The electron do- nor, i.e. the diamondoid, serves as “P-type” material.
Status of the patents: • U.S. provisional application US 61/006,801 filed on January 31, 2008
• PCT application WO 2009 / 099569 A1 filed on January 30, 2009
Contact: TransMIT GmbH, Dr. Kerstin Lischka, Chemist, Kerkrader Str. 3, D-35394 Giessen, Germany, Phone: +49-(0)641- 94364-25: http://www.transmit.de
http://www.hipo-online.net/files/Expose_Diamondoid_ Rectifier_TM290_EN_290310.pdf
11-01 :: December 2010 / January 2011
The figure depicts the tunneling current observed for a p- n-junction containing one diamondoid molecule. © TransMIT
Cyclopentadienylphosphazene Complexes (CpPN Complexes) of Metals of the Third and Fourth Group And of the Lanthanoids
Inventor(s): Prof. Dr. Jörg Sundermeyer, Dr. Konstantin Rufanov, Dr. Alexander Petrov, Michael Elfferding, Manuel Winkenstette
Abstract: The invention at hand provides cyclopentadienyl- phosphazene complexes (CpPN complexes) of the metals of the third and the fourth group and of the lanthanoids with the exception of lutetium for the first time. These complexes may be produced in situ, in which it is not required to make use of the partial- ly unstable alkyl compounds of these metals. Instead, the readily available and stable metal halides are utilised.
 Previous Page
Previous Page