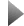66
nanotimes News in Brief
11-01 :: December 2010 / January 2011
Porphyrin Supramolecular Nanostructures // Controlled Assembly and Supramolecular Chirality
ZnTPyP could be controlled organized to form various well-defined nanostructures by means of a SAS, where an oil/ water system is employed as medium. © Dr. Penglei & team / Beijing National Laboratory for Molecular Science
R
esearchers from Institute of Chemistry, Chinese Academy of Sciences (ICCAS) have found that
a Zinc porphyrin (ZnTPyP) could be controlled assembled to form diverse nanostructures via a surfactant-assisted self-assembly (SAS) by using an oil/water system as medium.
In a general SAS process, organic units, which are dissolved in a guest solvent, are assembled with the assistance of surfactants that are dispersed in a host solvent. Commonly, the host and guest solvents have nice compatibility. Taking into account of the nice solubility of organic units in apolar or low-polar medium, a SAS using an oil/water system might be a subject of general interest. Now, Yunfeng Qiu, Penglei Chen and Minghua Liu have demonstrated that when a chloroform (oil) solution of ZnTPyP is added dorpwise to a CTAB aqueous solution (water), hollow nanospheres, solid nanospheres, nanotubes,
nanorods and nanofibers could be facilely synthe- sized depending on the concentration of the CTAB aqueous solution and the aging time. Moreover, the synthesized nanorods could be further hierar- chically organized to form regular nanoarray over large-area solid supports, while the others could not. Interestingly, although the employed ZnTPyP and CTAB species are achiral compounds, distinct supra- molecular chirality could be observed from the nano- rods, but could not from the other nanostructures.
Yunfeng Qiu, Penglei Chen and Minghua Liu: Evolution of Various Porphyrin Nanostructures via an Oil/Aqueous Medium: Controlled Self-Assembly, Further Organization, and Supramolecular Chirality, In: Journal of the American Chemical Society, Volume 132(2010), Issue 28, July 21, 2010, Pages 9644-9652, DOI:10.1021/ja1001967: http://dx.doi.org/10.1021/ja1001967
 Previous Page
Previous Page