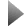56
nanotimes
News in Brief
and compounds containing ruthenium. Each has disadvantages.”
Their main disadvantage is cost and long-term availa- bility. Ruthenium-based solar cells can potentially be cheaper than silicon-based ones, but ruthenium is a rare metal on Earth, as rare as platinum, and will run out quickly when the demand increases.
Carbon is cheap and abundant, and in the form of graphene, capable of absorbing a wide range of light frequencies. Graphene is essentially the same stuff as graphite (pencil lead), except graphene is a single sheet of carbon, one atom thick. Graphene shows promise as an effective, cheap-to-produce, and less toxic alternative to other materials currently used in solar cells. But it has also vexed scientists.
For a sheet of graphene to be of any use in collecting photons of light, the sheet must be big. To use the absorbed solar energy for electricity, however, the sheet can‘t be too big. Unfortunately, scientists find large sheets of graphene difficult to work with, and their sizes even harder to control.
The bigger the graphene sheet, the stickier it is, making it more likely to attract and glom onto other graphene sheets. Multiple layers of graphene may be good for taking notes, but they also prevent electri- city.
By attaching a semi-rigid, semi-flexible, three-di- mensional sidegroup to the sides of the graphene,
Li and his collaborators were able to keep graphene
sheets as big as 168 carbon atoms from adhering
to one another. With this method, they could make the graphene sheets from smaller molecules (bottom-
10-04 :: April 2010
Image: Two graphene molecules (dark grey) are caged by side- groups (blue) attached to each graphene sheet. The sidegroups help prevent the graphene sheets from stacking, as they are prone to do. © Liang-shi Li
up) so that they are uniform in size. To the scientists‘
knowledge, it is the biggest stable graphene sheet ever made with the bottom-up approach.
The sidegroup consists of a hexagonal carbon ring and three long, barbed tails made of carbon and hydrogen. Because the graphene sheet is rigid, the sidegroup ring is forced to rotate about 90 degrees relative to the plane of the graphene. The three brambly tails are free to whip about, but two of them
 Previous Page
Previous Page