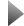11-11/12 :: November/December 2011
nanotimes News in Brief
49
The rectangular depression is the result of multiple bombardments of the surface with buckyballs and argon during a depth-profiling procedure. © Zbigniew Postawa, Jagiellonian University, Poland
other instances, using buckyballs alone makes for a bumpy surface on which to perform molecular depth profiling because the molecules can be distri- buted unevenly throughout the peaks and valleys,” Garrison explained. “In these instances, when low- energy argon bombardment is added to the process, the result is a much more even, smoother surface, which, in turn, makes for a better area on which to do analyses of molecular arrangement. In these cases, researchers can get a clearer picture of the many layers of molecules and exactly which mole- cules make up each layer.”
Zachary J. Schiffer, Paul E. Kennedy, Zbigniew Postawa, and Barbara J. Garrison: Molecular Dynamics Simulations Elucidate the Synergy of C60 and Low-Energy Ar Cobom- bardment for Molecular Depth Profiling, In: Journal of Physical Chemistry Letters, Volume 2, Issue 20, October 20, 2011, Pages 2635-2638, DOI:10.1021/jz201219x: http://dx.doi.org/10.1021/jz201219x
A team of scientists led by a Penn State University chemist has demonstrated the strengths and weak- nesses of a method of molecular depth profiling – a technique used to analyze the surface of ultra-thin materials such as human tissue, nanoparticles, and other substances. Team leader Barbara Garrison, Shapiro Professor of Chemistry and the head of the Department of Chemistry at Penn State University, explained that bombarding a material with bucky- balls (C60) is an effective means of molecular depth profiling.
Garrison‘s group found that, with buckyball bom- bardment alone at grazing angles, the end result is a very rough surface with many troughs and ridges in one direction. “In many instances, this approach works out well for depth profiling. However, in
Stanford researchers have used nanoparticles of a copper compound to develop a high-power battery electrode that is so inexpensive to make, so effi- cient and so durable that it could be used to build batteries big enough for economical large-scale energy storage on the electrical grid.
In laboratory tests, the electrode survived 40,000 cycles of charging and discharging, after which it could still be charged to more than 80% of its ori- ginal charge capacity. For comparison, the average lithium ion battery can handle about 400 charge/
 Previous Page
Previous Page