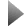74
nanotimes News in Brief
11-04 :: April/May 2011
Quantum Dots // Plasmonic Resonances in Semiconductor Nanocrystals
R
esearchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laborato-
ry (Berkeley Lab) have shown that plasmonic pro- perties can also be achieved in the semiconductor nanocrystals known as quantum dots. This discovery should make the field of plasmonics even hotter.
“We have demonstrated well-defined localized sur- face plasmon resonances arising from p-type carriers in vacancy-doped semiconductor quantum dots that should allow for plasmonic sensing and manipulation of solid-state processes in single nanocrystals,” says Berkeley Lab director Paul Alivisatos, a nanoche- mistry authority who led this research. “Our doped semiconductor quantum dots also open up the pos- sibility of strongly coupling photonic and electronic properties, with implications for light harvesting, non- linear optics, and quantum information processing.”
Alivisatos is the corresponding author of a paper in the journal Nature Materials. Co-authoring the paper were Joseph Luther and Prashant Jain, along with Trevor Ewers.
“Our study represents a paradigm shift from metal nanoplasmonics as we’ve shown that, in principle, any nanostructure can exhibit LSPRs so long as the interface has an appreciable number of free charge carriers, either electrons or holes,” Jain says. “By demonstrating LSPRs in doped quantum dots, we’ve extended the range of candidate materials for plas- monics to include semiconductors, and we’ve also
Transmission electron micrographs and (inset) showing the electron diffraction patterns of three quantum dot samples with average size of (a) 2.4nm (b) 3.nm, and (c) 5.8nm. © Alivisatos group
merged the field of plasmonic nanostructures, which exhibit tunable photonic properties, with the field of quantum dots, which exhibit tunable electronic properties.”
Jain and his co-authors made their quantum dots from the semiconductor copper sulfide, a material that is known to support numerous copper-deficient stoichiometries. Initially, the copper sulfide nanocry- stals were synthesized using a common hot injection method. While this yielded nanocrystals that were intrinsically self-doped with p-type charge carriers, there was no control over the amount of charge va- cancies or carriers.
“We were able to overcome this limitation by using a room-temperature ion exchange method to synthe- size the copper sulfide nanocrystals,” Jain says. “This
 Previous Page
Previous Page